Introducing MIMIX Technology
By Brian Hanley
Some of nature’s most fascinating creatures are those that have evolved to mimic others in order to survive and thrive. Chameleons may be the most well-known example; a reptile that can change colors to better blend in with its surroundings and avoid predators. Others – like the owl butterfly – use two eye-shaped spots on its wings to mimic an owl and scare off potential predators.
Here at Access Vascular, we are borrowing a page from nature’s playbook. Our patented MIMIX™ biomaterial technology mimics the human body’s natural chemistry to avoid detection in its highly evolved defense system.
This non-reaction is critical because our body’s defense mechanisms are effective at identifying and rooting out foreign invaders - from viruses to transplanted organs. In our research and development process, we realized this reaction was a critical factor behind the high incidence rate for vascular access complications.
Approximately 85%1 of hospital patients in the United States receive a vascular access device and roughly 30%2 of those encounter a vascular access-related complication. At best, these delay treatment, waste time for patients and hospital staff, and incur significant costs to our healthcare system. At worst, they lead to inordinate suffering, infection, and even death.3
These complications occur largely because nearly every intravascular catheter used in hospitals today is constructed of polyurethane or silicone, which are hydrophobic materials that repel water and are highly susceptible to protein adsorption. These properties signal to the body that it is a foreign substance and trigger its defenses, leading to clots (thrombosis) and infection. Even attempts to mask these materials using surface modifications and coatings have proven ineffective.4
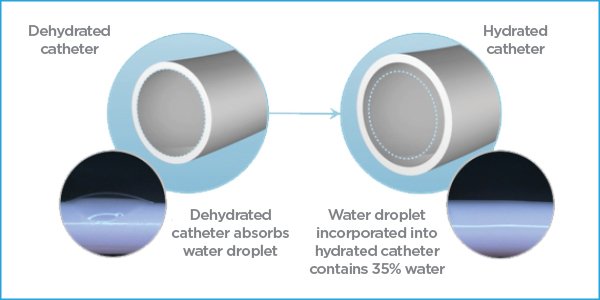
Realizing that the problem of most vascular access complications was not with a process but rather an imperfect material, we set to work figuring out how to bypass the body’s defense system. The result is MIMIX, a technology made from a composite hydrophilic material that absorbs water and thus evades the body’s sentinels.
Consisting of heat-treated polyvinyl alcohol, our biomaterial is a physically cross-linked high-strength biomaterial. Upon hydration this biomaterial absorbs water and the surfaces become consistently hydrophilic and lubricious. This surface makes it difficult for anything else to adhere to it. Additionally, cilia-like fibers are developed throughout the catheter during the manufacturing process. Acting similarly to cilia in the body, these water-loving fibers move around and search out water while pushing away anything else that tries to attach to the catheter. The result of these mechanisms of action is a device that repels protein adsorption and exhibits the ideal characteristics of thromboresistance, neatly sidestepping the body’s defenses.
The key difference between MIMIX technology and other catheter technologies that attempt to mask polyurethane – whether through additives in the base material or through surface coatings – is with MIMIX the entire composite catheter material is hydrophilic and contains no polyurethane or silicone. The MIMIX material in fact becomes superhydrophilic through the manufacturing process which refers to the phenomenon of water spreading to form a complete film on the catheter surfaces to maximize contact with water. As we bring this back to the human body, we know that 90% of blood plasma is water. Highly hydrophilic catheters that are designed to be non-adherent and have a water layer on the inner and outer surfaces can easily become camouflaged in the high water content of blood and thus avoid triggering the foreign body response that can lead to clotting and complications.
In in vitro testing, our HydroPICC and HydroMID biomaterial catheters, using MIMIX technology, have demonstrated an average of 97% less thrombus accumulation on their surface (based on platelet count) compared to standard polyurethane catheters.5 Clinical studies have shown MIMIX can significantly reduce, and in some cases, nearly eliminate routine vascular access complications.6,7
Another retrospective data review found that our midline catheters were able to remain in the body more than 2.5 times longer than polyurethane-based ones with significant improvements in failure rates and zero instances of Deep Vein Thrombosis (DVT).8 For hospitals using our HydroPICC and HydroMID catheters, a separate published economic analysis demonstrated that even with just a modest 50% reduction in complications, a typical 1000-bed acute care facility could save nearly $1.8 million dollars annually.4
Like many organisms in nature, MIMIX takes on the properties of its surroundings to elude detection. The result is a new type of catheter that can drastically reduce common (and sometimes dangerous) catheter complications and has the potential to save hospitals millions of dollars per year.
References
Keogh S, Flynn J, et al. Nursing and midwifery practice for maintenance of vascular access device patency. A cross-sectional survey. Int J Nurs Stud. 2015 Nov;52(11):1678-85. doi: 10.1016/j.ijnurstu.2015.07.001. Epub 2015 Jul 11. PMID: 26206327.
Grau, Delphine et al. (2017). Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: A prospective cohort study. Antimicrobial Resistance & Infection Control. 6. 10.1186/s13756-016-0161-0.
Central line-associated bloodstream infections (CLABSI). AHRQ. (n.d.). Retrieved December 20, 2022, from https://www.ahrq.gov/topics/central-line-associated-bloodstream-infections-clabsi.html#:~:text=As%20many%20as%2028%2C000%20patients,to%20prevent%20and%20reduce%20CLABSI.
Moureau NL (2022). International Journal of Nursing Health Care Research 5: 1347. DOI: https://doi.org/10.29011/2688-9501.101347
Data on file at Access Vascular. Reduction of thrombus accumulation was evaluated using in vitro and in vivo models. Pre-clinical in vitro/in vivo evaluations do not necessarily predict clinical performance with respect to thrombus formation.
Bunch, J. (2022). A Retrospective Assessment of Peripheral Catheter Failures Focusing on Catheter Composition. Journal of Infusion Nursing.
Bunch, J. (2021). Retrospective hydrophilic biomaterial PICC vs polyurethane PICC data presented at AVA 2021. Data on file at Access Vascular. Pending publication.